新着情報
<2012.12.11> 在シンガポール日本国大使館主催の日本天皇誕生日祝賀会が開催され、アジアメディカルセンターとしてレセプションに参加しました。
Celebration of the Birthday of His Majesty the Emperor
Venue: The Fullerton Hotel, Ballroom
Date: 11 Dec, 2012
Time: 18:30~20:00
<2012.11.28> Professor Malcolm Levittによる“SpinDynamica : A Mathematical Environment for Spin Dynamical Calculations”のセミナーがありました。
Speaker: Professor Malcolm Levitt
School of Chemistry, Southampton University, UK
Date : 28 November 2012
Time : 10.00am – 11.00am
Venue : SBIC Seminar Room, Biopolis

<2012.11.28> Dr. David Leavesleyによる“Wound Healing – more to it than meets the eye”のセミナーがありました。
Speaker : A/Prof David Leavesley
Director, International E&R, Biomedical Sciences,
Queensland University of Technology
Date : 28 November 2012
Time : 11:00am – 12:00pm
Venue : Breakthrough Theatrette, Matrix Level 4, Biopolis

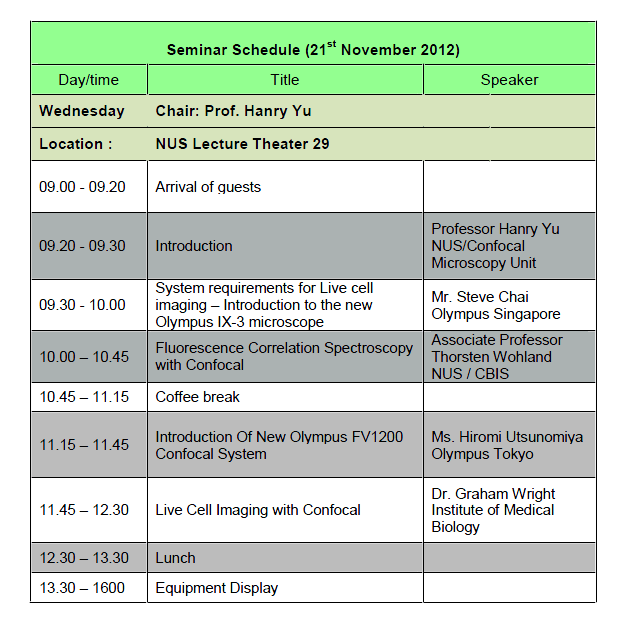
<2012.11.21> オリンパスの新しい2光子顕微鏡のプロモーションを兼ねオリンパス顕微鏡セミナーが開催されました。
Olympus Confocal and Live Cell Seminar
Date: 21st November 2012, 9am -12 pm
Venue: LT29, YLLSoM, National University of Singapore
Equipment Display 13:30- 16:00

<2012.11.07> Prof. Tan Seong-Sengによる " Hijacking cancer cell survival pathways to keep neurons alive after brain injury and its potential use for treatment"のセミナーがありました。
SPEAKER: Prof. Tan Seong-Seng
Date: 7 November 2012
Time : 11:00am
Venue: Level 3, IMCB Seminar Room 3-46, Proteos, Biopolis

<2012.11.01> GlaxoSmithKline (GSK)の長年のシンガポールへの貢献と更なるシンガポールとのパートナーシップの発展を祝うセレモーが開催されました。シンガポールからPrime Minister Lee Hsien Loongをはじめとする各機関の要人が、GSKからは、CEO Andrew Wittyが参加されました。
November 1, 2012
Singapore Prime Minister Lee Hsien Loong and GSK CEO Sir Andrew Witty lead celebrations at Commemoration Event
Quality Road site to receive S$40m to S$60m investment in sustainable manufacturing technology
SINGAPORE, Nov. 1, 2012 -- GlaxoSmithKline (GSK) today celebrates its long-standing relationship with Singapore by marking the 30th and 40th anniversaries of its Jurong and Quality Road manufacturing sites respectively. Singapore Prime Minister Lee Hsien Loong joined GSK CEO Sir Andrew Witty at a commemoration event held at GSK's Jurong site today, which recognised the key role the two sites have played in the evolution of GSK's extensive manufacturing organisation and the growth of Singapore's biomedical sector.
The partnership between GSK and Singapore has underpinned significant growth in GSK's business activities in Singapore. In addition to the production of active pharmaceutical ingredients (API) at Jurong and Quality Road, GSK has a number of additional operations in Singapore, including a Stiefel manufacturing facility at Gul Circle, research and development at Biopolis, a vaccines manufacturing facility at Tuas, and both regional headquarters and marketing and sales operations at Gateway.
GSK CEO, Sir Andrew Witty, commented, "GSK and Singapore have enjoyed a highly-productive partnership for more than 50 years, and with the continued support of the Economic Development Board (EDB) and our shared vision of improving the technical capabilities within the Singaporean biomedical science sector, this partnership has a very bright future. Our state-of-the-art manufacturing operations at both Jurong and Quality Road continue to produce many of our most established medicines, and looking to the future, they will also play a key role in delivering the innovative medicines in our late-stage pipeline."
EDB Chairman, Leo Yip also commented, "GSK's pioneering spirit and strong partnership with Singapore have been pivotal to the growth of the biomedical sciences sector in Singapore. Over the years, GSK has developed a deep pool of talent across different functions and pioneered new capabilities including sustainable manufacturing. Our heartiest congratulations to GSK on this significant milestone in the GSK-Singapore partnership and we look forward to building on this strong foundation. We are proud to be GSK's home in Asia."
GSK has set ambitious goals for driving sustainability in pharmaceutical manufacturing and distribution. Through a joint GSK-EDB S$50 million fund, it will enable Singapore to become a leader in sustainability research in pharmaceuticals and fine chemicals manufacturing. GSK is also investing an additional S$50 million into its "Factory of the Future" initiative, which seeks to reduce the company's carbon footprint through the use of a number of sustainable processes and green technologies.
In addition to this, GSK has recently committed to invest a further S$40-60 million in improving its antibiotic manufacturing capabilities at the Quality Road site. This enhanced process will utilise enzymatic technology to replace existing chemical processes during the production of one of its leading antibiotics, amoxicillin. This will enable GSK to maintain its high standards of product quality, but at a reduced cost and with a significantly lower environmental impact. GSK is committed to integrating environmental sustainability into its manufacturing and distribution networks in order to meet ambitious environmental targets -- the long-term goal is for GSK's entire value chain to become carbon neutral by 2050.
GSK has also developed key local talent across a number of disciplines in Singapore and the company continues its investment in both technical and business capabilities through the GSK-EDB Scholarship Programme and other local talent development initiatives.
As part of our celebration of 40 years of manufacturing in Singapore, GSK employees in Singapore raised S$120,000 to support three local charitable organisations -- the Children's Charities Association, the Singapore Cancer Society, and the Muscular Dystrophy Association of Singapore.
GlaxoSmithKline -- one of the world's leading research-based pharmaceutical and healthcare companies -- is committed to improving the quality of human life by enabling people to do more, feel better and live longer. For further information please visit www.gsk.com.
Notes to Editors
About GSK in Singapore
Singapore is home to GSK's Regional Headquarters (Emerging Markets & Asia Pacific), an R&D facility (Biopolis), two global API manufacturing and supply sites (Jurong & Quality Road), a Stiefel manufacturing facility, and a state-of-the-art vaccines plant (Tuas).
About Jurong and Quality Road Manufacturing Sites
The Pioneer Sector 1, Jurong plant was officially opened on 20th October 1982 by Dr Tony Tan Keng Yam, who was then Singapore's Minister for Trade and Industry. The plant was initially designed to make all five stages of Ranitidine Hydrochloride, the active compound for Zantac, a prescription medicine for the treatment of gastric ulcer. Since then, additional production capacity has been added to the site, including an R&D Pilot Plant. The site has evolved from bulk manufacture of established products in the early days to becoming a lead New Product Introductions (NPI) site within the GSK manufacturing and supply (GMS) network, focused on the development of late phase New Chemical Entities (NCEs) and delivering higher value products such as very active compounds in small batch sizes. The site currently has three production buildings making 12 Active Pharmaceutical Ingredients for a range of medicines prescribed for the treatment of respiratory, oncology, gastro-intestinal, allergy, anti-viral and neurological conditions.
The plant situated at Quality Road was built in 1972 and officially opened on 3rd May 1973 by then Finance Minister, Mr Hon Sui Sen. Built at a cost of over S$32 million, the plant sits on a five-hectare site. The site was originally built to produce a range of newly discovered semi-synthetic penicillins and had both primary and secondary production facilities to make the final dose forms. Over time, the site developed as the single source of Amoxcillin and Monosodium Ticarcillin within GSK, which are active compounds for the manufacture of Augmentin and Timentin, two of GSK's antibiotics widely prescribed for the treatment of a broad spectrum of bacterial infections. Currently there are over 590 personnel employed across the two facilities.
Cautionary statement regarding forward-looking statements
Under the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995, GSK cautions investors that any forward-looking statements or projections made by GSK, including those made in this announcement, are subject to risks and uncertainties that may cause actual results to differ materially from those projected. Factors that may affect GSK' s operations are described under 'Risk Factors' in the 'Business Review' in the company' s Annual Report on Form 20-F for 2007.
Source: GlaxoSmithKline
<2012.10.30> The Postgraduate Medical Institute of the Singapore General Hospitaでは、SGHスタッフだけでなく、他機関の医療従事者のトレーニングコース参加も可能になりました。
The Postgraduate Medical Institute of the Singapore General Hospital (SGH-PGMI)では、SGHスタッフだけでなく、他機関(一部は海外も含む)の医療従事者のトレーニングコース参加も可能になりました。継続的な医療技術の向上と最先端医療情報&技術の獲得が可能です。
http://www.sgh.com.sg/subsites/pgmi/pages/home.aspx
<2012.10.29~30> Scientific Conference "Innovative chemical & Engineering Solutions for Sustainable Growth"が開催されました。
Scientific Conference "Innovative chemical & Engineering Solutions for Sustainable Growth"が開催されました。31日にはInstitute of Chemical and Engineering Scienceのラボツアーもありました。
http://www.icesconference.com.sg/index.php
日本からは、Dr Terunori Fujita, President & Chief Executive Officer, Mitsui Chemicals Singapore R&D Centre Pte Ltdの講演がありました。
<2012.10.25> シンガポール科学技術研究庁(A*STAR)と大阪商工会議所が医療機器産業に携わる日本企業のためにMOUを締結しました。
Press Releases
Thursday, October 25, 2012
Tokyo, Japan, 25 October 2012: Osaka Chamber of Commerce and Industry (OCCI), the key non-profit organization promoting medical industry in Japan, has inked a Memorandum of Understanding (MOU) with Singapore’s Agency for Science, Technology and Research (A*STAR). The MOU signals OCCI’s intent to foster and strengthen their partnership with Singapore in developing medical technology (MedTech).
2. The MOU will provide opportunities for Japanese MedTech companies to tap on Singapore’s robust R&D infrastructure and talent. Japanese MedTech firms will be able to fast-track research and product development of medical technology and devices for the Asian population, while leveraging on Singapore as a base to eventually launch their products into the region. The MedTech sector currently accounts for approximately US$336 billion in annual worldwide revenues.
3. Speaking on the collaboration, Dr Tan Sze Wee, Deputy Executive Director Biomedical Research Council and Director Healthcare and Lifestyle of A*STAR, said, “This partnership between A*STAR and OCCI comes at an opportune time with the increasing research interest in the development of medical technology equipment and devices for diseases affecting the Asian population. I am confident that A*STAR’s multi-disciplinary capabilities and ability to translate bioengineering research into innovative MedTech products will help Japanese MedTech companies to develop novel medical technology products to meet today’s complex healthcare challenges and enhance lives.”
4. Dr Yoshiyuki Taenaka, Chairman, Osaka Chamber of Commerce and Industry Medical Device Forum, said "As the largest economic organisation in Western Japan, OCCI represents the interests of many Japanese MedTech companies in Japan. The MOU will allow many of such MedTech firms to benefit from A*STAR's core capabilities in R&D, as well as Singapore's strong connection to both the East and the West, for MedTech product development."
_____________________________________________________________
For media queries and clarifications, please contact:
Mr. Wilson Xie
Agency for Science, Technology and Research
Senior Officer, Corporate Communications
Tel: (+65) 6826 6262
Mobile: 98775483
Email: wilson_xie@a-star.edu.sg
Ms. Ako Makiyama
Osaka Chamber of Commerce and Industry
Deputy Director
Economy and Industry Division
Manager
Life Science Group
Tel: (+81) 6 6944 6484
Email: makiyama@osaka.cci.or.jp
_____________________________________________________________
About the Agency for Science, Technology and Research (A*STAR)
The Agency for Science, Technology and Research (A*STAR) is Singapore's lead public sector agency that fosters world-class scientific research and talent to drive economic growth and transform Singapore into a vibrant knowledge-based and innovation driven economy.
In line with its mission-oriented mandate, A*STAR spearheads research and development in fields that are essential to growing Singapore’s manufacturing sector and catalysing new growth industries. A*STAR supports these economic clusters by providing intellectual, human and industrial capital to its partners in industry.
A*STAR oversees 20 biomedical sciences and physical sciences and engineering research entities, located in Biopolis and Fusionopolis as well as their vicinity. These two R&D hubs house a bustling and diverse community of local and international research scientists and engineers from A*STAR’s research entities as well as a growing number of corporate laboratories.
Please visit www.a-star.edu.sg
About the Osaka Chamber of Commerce and Industry (OCCI)
Osaka Chamber of Commerce and Industry (OCCI) is a comprehensive regional economic organization with membership consisting of all voluntary members. Supported by respectable members of various scales with wide-ranging lines of business, the OCCI represents the Osaka-Kansai region as a major economic organization.
The mission of OCCI is to contribute to the realization of a revitalized economic society, reconversion of industrial structure, enhancement of international competitiveness, renovated business management by member companies and creation of business opportunities.
Especially OCCI has been making special efforts in the promotion of life-science related industries as a driving force in boosting local economy. We promote exchange activities among member companies of great variety and also positively act as a bridge between industry and academia. We have network of academic circles and industries of nation-wide area. We also are focusing on creating an environment that promotes MedTech business in Osaka.
<2012.10.22~23> Workshop on Live Cell ImagingがNUSにて開催されました。
Workshop on Live Cell Imaging (Confocal Microscopy Unit)
Talk on Live Cell Imaging with Confocal Microscope
(By Prof. Miwako Ozaki)
Date: 22 October 2012 (Mon), Time: 10am to 12am
Venue: MD11 Symposium room 1 (all are welcome)
One-day Workshop on IX83, FV10i & FV1000
Date: 23 October 2012 (Tue)
Time: 9.30am to 5.30pm
Venue: MD11 #04-04
(only 15 slots available for the workshop, do register early!)
Please indicate your interest in either or both sessions by clicking on this link: https://esurvey.nus.edu.sg/efm/se.ashx?s=1CC7023E3E722F64




