2016.03.03セミナー
3月開催 Industry Professionals Seminarsのお知らせ
企業人専門家向けの医療機器に関する国際規格に関するセミナー
[Introduction]
Medicals Medical Technology, or MedTech, is emerging as one of the fast-growing industrial sectors worldwide, covering a wide range of applications from medical devices, equipment, and apparatus to orthopaedics, diagnostics, and medical consumables. In Singapore, MedTech manufacturing has been identified as one of the key manufacturing sectors. As part of Singapore’s growing biomedical sciences sector, the medical technology industry almost tripled its manufacturing output from S$1.5 billion in the year 2000 to about S$4.3 billion in the year 2011. Over the same period, its manpower base more than doubled – from about 4,000 to 9,000. By the year 2015, the medical technology sector is targeting to achieve S$5 billion in manufacturing output. In the next decade alone, the MedTech market is expected to grow rapidly creating huge business opportunities for local companies. As one of the most regulated industrial sectors, it is essential that industrial players gain necessary knowledge on MedTech product regulations and skills related to product design, manufacturing processes, and management techniques unique to this niche sector.
[Why MINDS Seminars?]
Supported by the Singapore Workforce Development Agency (WDA), SMF MINDS will equip MedTech companies with critical and much-needed skills across the medical device product life-cycle from product development to distribution. The series is relevant for small and medium-sized enterprises (SMEs) as well as non-SMEs, and is targeted at business entrepreneurs, middle management professionals and technical specialists and other occupations.
[Benefits of MINDS Seminars]
Through SMF MINDS, companies will be empowered to develop a better skilled workforce to conceptualise and introduce innovative medical devices and enhance their competitiveness in both regional and international markets. The seminar series featured a strong pipeline of speakers from overseas and local industry experts, such as design control in medical device product development, quality management system ISO13485, Singapore product registration know-how 101 as well as product distribution and business strategy.
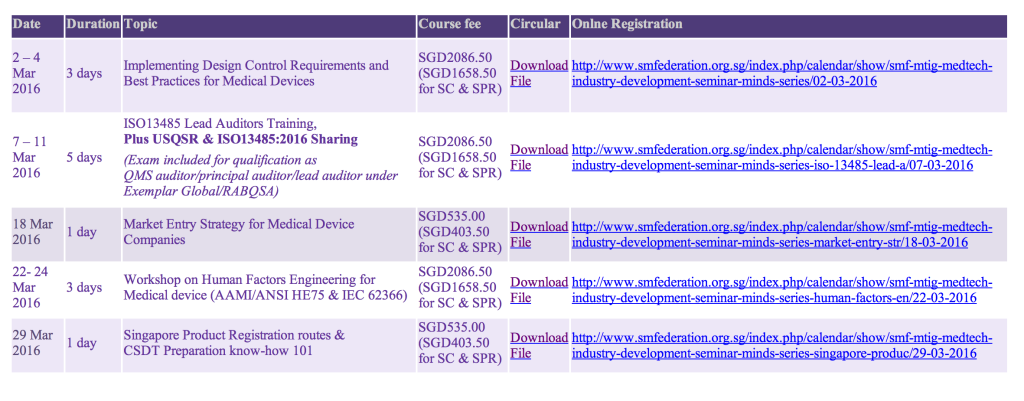
[MINDS Seminars Registration]
Limited seats available for WDA Subsidy.
Please register early to receive WDA Subsidy.
Thank you for your attention.